Ultrasonic Cleaners
Qualifying a Sonicator Bath for Sample Preparation
In the pharmaceutical, cosmetic and food processing industries ultrasonic cleaners are employed as sonicator baths for sample preparation for analytical testing. Unlike cleaning medical devices, when using an ultrasonic bath to prepare samples,the analytical method that uses ultrasound is validated, not the equipment. Guidelines include ICH Q2(R1), procedures for validating analytical methods, and USP 1225, validating…
Validating Ultrasonic Cleaners in a CGMP Environment
Ultrasonic cleaners play critical roles as tools to clean small parts such as pumps, valves and filters used in drug development and manufacturing. As such, ultrasonic cleaners can be validated in CGMP environments and in Title 21 of the Code of Federal Regulations as a processing tool delivering energy, called cavitation, to clean equipment. This…
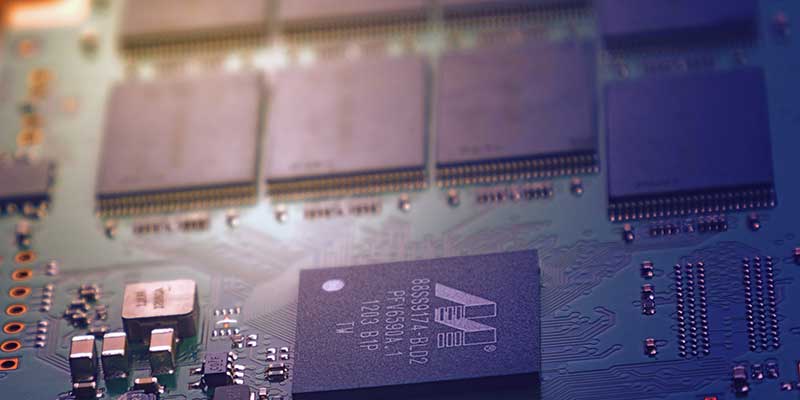
The Ultimate Guide to Ultrasonic PCB Cleaning: Processes, Chemicals and Oxidation Removal
The main challenges to how to properly cleaning a PCB are fragility and complexity. This makes manual cleaning with brushes, flammable solvents such as IPA, other solvent sprays and water jets impractical and potentially dangerous. Without doubt, the best cleaner for PCBs is an ultrasonic cleaner. This is why PCB manufacturers employ the technology to…
The Ultimate Guide to Ultrasonic Laboratory Equipment Cleaning
Learn how ultrasonic energy applied to lab equipment cleaning helps ensure compliance with FDA & CGMP regulations. FDA guidelines focus on eliminating cross-contamination and ensuring the integrity of analytical results. For many labs, achieving Current Good Manufacturing Practice (CGMP) standards means moving beyond manual scrubbing. Ultrasonic lab equipment cleaners when paired with the correct ultrasonic…
Picking the Best Industrial Ultrasonic Cleaner
The best industrial ultrasonic cleaner is one capable of removing tenacious contaminants and operating over long periods of time, regardless of part size. For parts such as automotive engine transmissions, a large industrial ultrasonic cleaner will be necessary. When selecting any industrial ultrasonic cleaner for your requirements, the first criterion to consider is the tank…
Specifying an Ultrasonic Cleaner
Specifying an ultrasonic cleaner for a specific application requires careful consideration of several key factors to ensure effective and efficient cleaning. The process involves more than just selecting a machine; it necessitates a comprehensive understanding of the technology, the cleaning task at hand, and the proper operational procedures. Before proceeding further, first determine how you…
How to Select the Best Ultrasonic Cleaning Solution
Choosing the correct ultrasonic cleaning solution chemistry is arguably the most critical step in the entire ultrasonic cleaning process, directly determining the effectiveness, safety, and efficiency of the operation. That’s because the wrong chemistry can fail to remove contaminants, damage the parts being cleaned, or even pose a hazard to the operator. This post will…
Ultrasonic Carburetor Cleaner Commonly Asked Questions
Ultrasonic Cleaning Vinyl Phonograph Records
According to Business Research Insights, the global vinyl records market was valued at $0.36 billion in 2024, and projected to grow to $1.29 billion in 2033. Increasing popularity of “new” vinyl records is matched by collectors looking for “old vinyl.” Depending on a record’s condition, artist, genre, and other factors, “old vinyl” can have values…
Safe Cleaning Fine Mesh Lab Sieves
Lab sieves, according to Thomas Scientific, are essential tools for particle size analysis in various industries, including pharmaceuticals, chemicals, and construction. These sieves are designed to separate and classify particles by size, ensuring consistency and quality in your products and samples. Lab test sieves that include an NIST traceable calibration certificate , designed to meet…